Rationale for Community Action & the Vashon Island Strategy
 Rural and tribal communities are at substantial risk from the COVID-19 pandemic caused by the coronavirus
SARS-CoV2 pandemic (CNN,
NYTimes,
Vox).
Several factors contribute to this risk, including a paucity of acute care resources, long drive times to access
care, an aging population often with complicating health issues, and multi-generational homes with a higher
proportion of essential workers when compared with urban centers (Politico).
Geographical isolation may delay the arrival of COVID-19 in rural vs urban areas, but that isolation has thus
far been associated with even greater delays in essential responses- social distancing measures and testing for
the virus followed by vigorous contact tracing.
Rural and tribal communities are at substantial risk from the COVID-19 pandemic caused by the coronavirus
SARS-CoV2 pandemic (CNN,
NYTimes,
Vox).
Several factors contribute to this risk, including a paucity of acute care resources, long drive times to access
care, an aging population often with complicating health issues, and multi-generational homes with a higher
proportion of essential workers when compared with urban centers (Politico).
Geographical isolation may delay the arrival of COVID-19 in rural vs urban areas, but that isolation has thus
far been associated with even greater delays in essential responses- social distancing measures and testing for
the virus followed by vigorous contact tracing.
Vashon is a rural island community of 11,000 located in Puget Sound near Seattle, WA. We have no acute care
or hospital care. When faced with these challenges, we developed and deployed a testing strategy that allowed our community to launch a successful testing
site in less than 3 weeks using local resources, a volunteer workforce and without resources from our
county’s public health system that was already stretched thin. We believe that by following our blueprint, rural
and tribal communities could stand up similar testing in one week using volunteer or professional medical and
non-medical staff.
Our strategy is founded on three basic principles: 1) that informed patients can self-collect nasal swab
specimens for testing; 2) that exposure of volunteer healthcare workers to SARS-CoV2 must be minimized; and 3)
PPE use can be minimized during testing by isolating patients and workers from each other throughout the testing
process.
 The most important element of this strategy is the reliance on patients to swab their own noses by following
clearly written instructions delivered before they come to the test site and immediately before swabbing their
noses. This method was pioneered by the Seattle
Flu Study, the SCAN project and a recent 500-patient prospective study
comparing side-by-side self-collected anterior nares (nasal) to medical technician-collected nasopharyngeal (NP)
swabs. This study demonstrated 94% sensitivity of the nasal swab strategy, leading to FDA
approval of this method on March 23, 2020. In a recent
opinion piece, Bill Gates listed self-collected nasal swab collection of COVID-19 specimens as the 1st
of five innovations essential for reopening of the US economy.
The most important element of this strategy is the reliance on patients to swab their own noses by following
clearly written instructions delivered before they come to the test site and immediately before swabbing their
noses. This method was pioneered by the Seattle
Flu Study, the SCAN project and a recent 500-patient prospective study
comparing side-by-side self-collected anterior nares (nasal) to medical technician-collected nasopharyngeal (NP)
swabs. This study demonstrated 94% sensitivity of the nasal swab strategy, leading to FDA
approval of this method on March 23, 2020. In a recent
opinion piece, Bill Gates listed self-collected nasal swab collection of COVID-19 specimens as the 1st
of five innovations essential for reopening of the US economy.
Healthcare
workers are at particular risk of contracting COVID-19 because of close and repeated contact with
patients that may be infectious. This is especially true during acquisition of nasopharyngeal samples
which often induces sneezing that may aerosolize the virus. In rural and tribal communities staffing of
clinics is often limited in the best of times: limiting healthcare workers exposure is essential. Our
drive-through testing strategy is designed to minimize exposure of workers to patients and patients to workers.
By having patients self-collect specimens in their car, we are able to maintain a safe distance between
patients and workers while still observing the swabbing process and being available if/when problems
arise. This process also minimizes PPE required to keep medical and non-medical workers safe.
Creating a Community COVID-19 Testing Site
Set up a Steering Committee
A committee of 5 or 6 people is needed that bring a range of expertise including medical professionals
(infectious diseases if available), a clinic manager or someone with program management experience and someone
who can organize logistics support including IT. The group should quickly choose a leader who can represent the
project in discussions with other emergency management organizations. This effort requires a major time
commitment- the group will need to meet daily at first and work together collaboratively, potentially for
months. An advisory committee (infectious disease, public health, local clinicians) will improve
decision-making, networking, and chain-of-command reporting, while reducing time commitments for rural
clinicians and public health officials already over-burdened by the pandemic.
Work with the Incident Command Team Coordinating the COVID-19 Response (local, county, state,
federal)
 The COVID-19 epidemic has put the US public health system under enormous strain, a fact that is exacerbated by
chronic under-funding. Worldwide travel patterns introduced the novel Caronavirus into urban areas where
high population densities helped it to spread. As a result, most public health efforts have so far been
concentrated in cities. We believe that rural communities can and should accept responsibility for protecting
their communities now. We suggest engaging and marshaling local resources while communicating your
intentions upward. If your community or area has a Medical Reserve
Corp (MRC), begin by talking to them- they may already be organizing. If your community has a disaster
preparedness organization talk to them- they will have an Incident Command Team. Your Testing and Tracing
site will become an integral part of your community’s response to the pandemic, and must necessarily
integrate into your community’s incident response structure. The Vashon MRC is housed within a 501(c)(3)
disaster preparedness organization (VashonBePrepared) that
provides logistics, communications and fund raising support for the MRC testing effort in addition to many other
community support activities.
The COVID-19 epidemic has put the US public health system under enormous strain, a fact that is exacerbated by
chronic under-funding. Worldwide travel patterns introduced the novel Caronavirus into urban areas where
high population densities helped it to spread. As a result, most public health efforts have so far been
concentrated in cities. We believe that rural communities can and should accept responsibility for protecting
their communities now. We suggest engaging and marshaling local resources while communicating your
intentions upward. If your community or area has a Medical Reserve
Corp (MRC), begin by talking to them- they may already be organizing. If your community has a disaster
preparedness organization talk to them- they will have an Incident Command Team. Your Testing and Tracing
site will become an integral part of your community’s response to the pandemic, and must necessarily
integrate into your community’s incident response structure. The Vashon MRC is housed within a 501(c)(3)
disaster preparedness organization (VashonBePrepared) that
provides logistics, communications and fund raising support for the MRC testing effort in addition to many other
community support activities.
I just finished the Test and wanted to thank you all for a job so well done. Superb planning, friendly helpers, you all made it work like clockwork! Thank you!”
You should quickly let your county or state public health department know what you want to do. They may be able
to help with resources and are likely to be supportive of your activities because it relieves them of the burden
of providing COVID-19 testing in your community. They may or may not be supportive of patient self-collection of
samples, but you have an opportunity to educate them about this FDA approved method. Explain its larger
benefits.
Test Site and Logistics
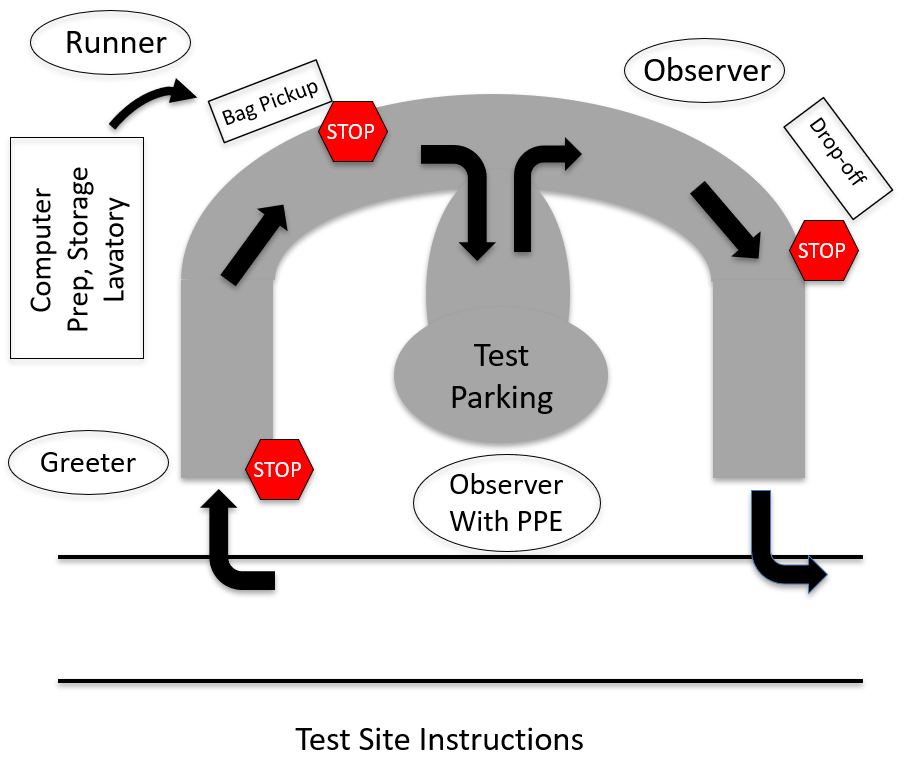 A generic site map is available. Our test site is located at a historical home on public land that has a
modest sized parking area adjacent to a semicircular drive. Desktop computers, sampling materials, PPE, and test
site supplies are set up and stored in the home's garage. There is ready access to lavatory and separate
hand-washing facilities.Flow through the site is as follows:
A generic site map is available. Our test site is located at a historical home on public land that has a
modest sized parking area adjacent to a semicircular drive. Desktop computers, sampling materials, PPE, and test
site supplies are set up and stored in the home's garage. There is ready access to lavatory and separate
hand-washing facilities.Flow through the site is as follows:
- Patients stop at one end of the drive and are met by the Greeter. Patients show their ID through a
closed car window and the Greeter confirms their appointment.
- A Runner brings sampling materials and instructions contained in a disposable paper bag, and places them on
a table to which the patient is directed once the Runner has moved away.
- The patient stops and picks up the bag, and is then directed by the Runner to a parking space.
- The patient reviews instructions and swabs their
nose under supervision by a Medical Observer who is wearing PPE. Patient then places sample vial in
biohazard bag that also contains a lab requisition in an exterior sleeve.
- Patient is directed to the drop off point and deposits their sample in a cooler containing frozen ice packs.
Patient then leaves the site.
- At the end of the day, the contents of the cooler are double bagged by the medical volunteer in PPE for
courier pick-up. Usually a single set of gown, gloves, mask and eye protection are needed per 4-hour
shift.
While the test site needs are simple, there is a requirement for lots of things that are essential but are also
relatively easy to find in most communities. A complete list can be found here.
The Test Site Workforce
 The testing strategy we use could be carried out in association with an established clinic or could operate
independently staffed entirely by volunteer healthcare and other workers. The process is straight forward
enough that any motivated organization could set this up in collaboration with a clinic or volunteer medical
organization.
The testing strategy we use could be carried out in association with an established clinic or could operate
independently staffed entirely by volunteer healthcare and other workers. The process is straight forward
enough that any motivated organization could set this up in collaboration with a clinic or volunteer medical
organization.
Our site operates with a staff of 2 non-medical and 2 medical volunteers. Non-medical personnel carry out
Greeter and Runner functions. Our runner also directs cars from pick-up to parking and parking to drop-off
locations. One medical volunteer in PPE is the test observer and is responsible for collecting and preparing
samples for shipping at the end of the testing day. A second medical volunteer manages the schedule in real time
and has responsibility for assembling sampling materials & instructions. As we have gained experience,
we can operate the site with 2 non-medical volunteers (the Runner also observes drop off) and 1 medical
volunteer (if new patients are not added during testing hours).
Our efforts to minimize exposure of workers has been critical to recruitment efforts, as was training of
volunteers before the site opened to minimize exposures and understand appropriate use of PPE.
COVID-19 Testing Strategy
Sampling by Self-Collected Nasal Swab
 It is important that patients understand the importance of careful collection of the nasal specimen. It
isn’t complicated, but we believe it is important to use instructions that have been proven to work. For
this reason, we provide the same written patient
instructions (with permission) that were used in the Everett WA study. We reinforce the importance of
reading and following the instructions exactly during a pre-test conversation during which the test is
scheduled.
It is important that patients understand the importance of careful collection of the nasal specimen. It
isn’t complicated, but we believe it is important to use instructions that have been proven to work. For
this reason, we provide the same written patient
instructions (with permission) that were used in the Everett WA study. We reinforce the importance of
reading and following the instructions exactly during a pre-test conversation during which the test is
scheduled.
Who to Test for COVID-19
Current guidelines in WA and across the nation, limit testing to symptomatic patients with a healthcare
provider’s order. Initially, testing was limited to those at high risk from COVID-19 due to age or
other risk factors (diabetes, lung, heart or kidney disease, immunodeficiency and pregnancy). As testing
has become more available, guidelines have been relaxed so that testing of anyone with symptoms of fever, upper
or lower respiratory complaint or body aches and chills is now warranted. It is likely that testing criteria
will change, so it is wise to check the FDA
and CDC COVID-19 websites
frequently for new information.
We facilitate provider orders by including an order form on our COVID-19 testing website. However, ~10% of our patients do
not have a primary care provider. In that circumstance, Vashon MRC health providers screen patients and decide
whether a test is warranted. It is essential when providing this service to clearly communicate whether or not
you can provide management of tested patients’ symptoms. We do not have that capability (we’re not a
clinic), so we provide web links and phone numbers for low cost clinics in our area.
Personal Protective Equipment
 Our non-medical workers wear disposable or cloth masks as suggested by our local health department for all people
when in public. We provide gloves if workers want to wear them. It should be emphasized during training that if
gloves are worn, hands should still be washed regularly- a contaminated glove is just as effective in spreading
COVID-19 as a naked hand.
Our non-medical workers wear disposable or cloth masks as suggested by our local health department for all people
when in public. We provide gloves if workers want to wear them. It should be emphasized during training that if
gloves are worn, hands should still be washed regularly- a contaminated glove is just as effective in spreading
COVID-19 as a naked hand.
One medical worker who supervises patient self-collection of nasal specimens wears an N95 mask and has ready access to a gown gloves and face shield on the rare occasion that they are needed to help a patient with the test. This worker does not need to change PPE during a shift unless they come in
close contact with a patient. At the close of the testing day this worker collects samples and prepares them for
shipping before disposing gown and gloves. N95 masks are reused. A 2nd medical worker mans a computer station and does not
require PPE beyond a cloth or disposable mask.
 Social distancing is practiced by all test-site workers. This is most challenging in the enclosed space we
use for scheduling and materials storage during preparation of sampling bags before the testing day begins and
during computer training of individuals learning our scheduling system.
Social distancing is practiced by all test-site workers. This is most challenging in the enclosed space we
use for scheduling and materials storage during preparation of sampling bags before the testing day begins and
during computer training of individuals learning our scheduling system.
Testing Laboratory
The acquisition of sampling materials and selection of a diagnostic lab to perform the RT-PCR test is
critical. We chose LabCorp because they could supply us with sampling materials, already provided courier
service to our rural community, had the lowest price we could find, are willing to bill patients’
insurance, and return results through a convenient web interface in 48-72 hours. Their service has been generally
very good. If a commercial laboratory already services a local hospital or clinic and provides COVID-19 RT-PCR
testing, that may be the easiest way to get up and running quickly.
Below is a list of large commercial clinical diagnostic laboratories that can perform molecular diagnostic
testing on nasal specimens with contact information, list price and unverified turnaround times. You may
be able to negotiate a better price than the list price by calling and negotiating, particularly if you
anticipate large number of tests.
You should also talk to your local health department about testing supplies. If you are able to locate your own
sampling materials-synthetic swabs (no cotton, wood shafts or Ca-Alginate) and transport medium) you will have a
larger choice of clinical laboratories.
Tracking Samples and Results
 If you are a clinic or working with a clinic you probably have access to an electronic medical record and
scheduling tools. This is a major advantage because these tools are generally more user friendly than the
scheduling process we use and they will already be HIPAA compliant.
If you are a clinic or working with a clinic you probably have access to an electronic medical record and
scheduling tools. This is a major advantage because these tools are generally more user friendly than the
scheduling process we use and they will already be HIPAA compliant.
Because VashonBePrepared's MRC COVID Testing Project is not associated with a clinic, we put together a spreadsheet-based
system for tracking samples and patient contacts. We found it most convenient to use the G-Suite of tools because they are sufficiently secure to allow HIPAA compliance. The system is comprised
of several components.
First the website we provide in the Toolkit contains a web submission form that providers can use to submit
orders. The output of this submission is a g-sheet containing provider and patient contact information. This is how most orders come to us.
As orders arrive, we first check that the provider’s NPI number
is valid as a control against patients ordering tests. This has not been a problem. We then
call the patient to collect demographic, contact and insurance information. This information is entered in a
Patient Information Form that serves as a requisition
for the diagnostic lab. We also ask about common COVID-19 symptoms so that appropriate ICD-10 codes
can be included.
We then ask the patient when they would like to come to be tested and transfer patient and provider information
into a Patient Schedule G-sheet. Each patient visit
is assigned a unique identifier so we don’t have to use their name at the testing site. This number
consists of Date-Time-Lane: e.g. 421-1300-1 is a time slot on April 21 at 1pm. The lane number allows
the schedule to be double or triple booked if testing is parallelized to increase capacity. Anyone
with access to the scheduling G-sheet can modify it, so most of our scheduling is done from the
scheduler’s home. A confirmation of test time is emailed to the patient along with driving
instructions to the site, a description of
site logistics, instructions for nasal swabbing and a “What you need to know” document.
Finally, after the test day is complete, patient and provider information are copied onto a Master Patient List
that allows us to manage
reporting of test results to providers and patients. This master list is downloaded to an external
storage device nightly to provide a secure backup of all tests performed and their status.
Test Reporting and Contact Tracing
 Results should be returned in less than 72 hours and should be reported back to providers and patients
quickly. We check for results on the LabCorp information system (and update the Patient Master List)
several times a day. We use the LabCorp system to email or fax patient results directly from their
system. Some labs may already have this set up for the clinics and providers they serve, so check with
them.
Results should be returned in less than 72 hours and should be reported back to providers and patients
quickly. We check for results on the LabCorp information system (and update the Patient Master List)
several times a day. We use the LabCorp system to email or fax patient results directly from their
system. Some labs may already have this set up for the clinics and providers they serve, so check with
them.
We believe it is important to speak with patients whether their result is positive or negative and have developed
scripts for reporting of both positive and negative results to patients. The focus of the discussion with positive patients is reinforcing the importance of isolation, identification of needed support services and
identifying potential contacts. The focus of discussion with negative patients is the possibility of a
false negative result and the need for continued isolation for a total of 14 days from the onset of symptoms or
until symptoms have been gone for at least 72 hours, whichever is longer. We recognize this is a
conservative recommendation, but believe it is warranted because we do not know with certainty what the true
false negative rate of nasal or nasopharyngeal swabbing is.
You should contact your county or state public health department to understand what support they can provide for
contact tracing. They are likely to be glad for the help! Contact tracing is just beginning in King County
after 6 weeks in which there was too much disease to be able to do it effectively. We are working with our local
health authorities to develop the precise mechanism and scripting for this activity. This site will be
updated with scripting and further advice when it becomes available.
Public Outreach and Information
Webpage Template
 A concise webpage is the quickest way to communicate information about your testing activity to your community.
We provide generic content that describes the testing strategy and related information. This sample webpage is
available for your use in three formats:
A concise webpage is the quickest way to communicate information about your testing activity to your community.
We provide generic content that describes the testing strategy and related information. This sample webpage is
available for your use in three formats:
- Bare HTML, with no external dependencies
- Simple styling, using Bootstrap4, jQuery, jQueryUI, and Google Fonts open source libraries
- Fancier styling, with images and Fontawesome that can serve as a standalone website, perhaps as a subdomain
to your own site.
These can be downloaded and modified as desired from www.github.com/EOConline/covid-testing. Limited support is available through RT3@VashonBePrepared.org,
or by filing an issue on GitHub.
Legal Considerations
HIPAA Requirements Need to be Met
It is important that patients’ protected health information (PHI) is handled in a HIPAA compliant fashion.
This means ensuring your information system conforms and that people who have access to PHI treat it appropriately. Training of volunteers
is advised. Several common errors to be avoided are:
- Storing PHI on your personal computer
- Not signing out of your information system when work is done
- Sending PHI through unencrypted email servers
- Leaving a message on a patient’s answering machine without prior approval
- Delivering PHI to incorrect fax numbers, email addresses or phone numbers.
If you plan to have providers submit orders from your website, be sure to use https rather than http. This level
of security is required for HIPAA compliance.
Liability Protection from States, CARES Act Coverage
Most states provide liability protection in the form of immunity for volunteer healthcare workers responding to a
declared emergency. Moreover, the CARES act (SEC
4216), passed in response to the COVID-19 emergency, provides that volunteer healthcare professionals engaged in
the emergency response to COVID-19 “shall not be liable under Federal or State Law for any harm caused by
an act or omission of the professional in the provision of health care services during the public health
emergency declared by the Secretary of Health and Human Services.”
We recommend that anyone standing up a testing site consider obtaining legal advice to understand the limits of
this and any other liability coverage.
Who We Are
The Vashon COVID-19 Testing Project Steering Committee
 Jim Bristow, MD (Chair) is a semi-retired physician-scientist living on Vashon Island. He is a graduate of
Harvard Medical School and was a practicing pediatric cardiologist at UCSF until retirement in 2015. From
2004-2015 he served as Deputy Director of the Department of Energy’s Joint Genome Institute and is currently a
senior advisor to the Biosciences Area at Lawrence Berkeley National Laboratory. He is a new recruit to the
Vashon MRC.
Jim Bristow, MD (Chair) is a semi-retired physician-scientist living on Vashon Island. He is a graduate of
Harvard Medical School and was a practicing pediatric cardiologist at UCSF until retirement in 2015. From
2004-2015 he served as Deputy Director of the Department of Energy’s Joint Genome Institute and is currently a
senior advisor to the Biosciences Area at Lawrence Berkeley National Laboratory. He is a new recruit to the
Vashon MRC.
Zach Miller, MD is a graduate of Columbia University College of Physicians and Surgeons. He did post graduate
training in Pediatrics at Stanford and Infectious Diseases at Children's Hospital in Seattle. From 1977 to 2015
he worked as an Infectious Diseases specialist at Group Health/Kaiser where he pioneered home antibiotic
treatment and other patient self-care strategies. He served on the Group Health Permanente Medical Group Board
of Directors until his retirement in 2015.
Ina Oppliger, MD is a board certified rheumatologist. She completed medical school and internal medicine
residency at the University of Kansas, followed by a fellowship in Rheumatology at the University of Washington.
She was a clinical associate professor at the University of Washington and staff physician at Group Health
Permanente, Medical Group where she served on the Board of Directors. She is the co-director of the Vashon MRC
and a Pierce County MRC volunteer for the past 2 years.
Bonny S Olney, DO received her degree from The University of North Texas Health Science Center and trained in
Family Practice at the University of Texas Health Science Center, Houston followed by 20 years in independent
family practice. She worked as an IPA medical director for 2 years helping independent physicians navigate
value-based care and helped launch a Medicare Accountable Care Organization. She currently works as a Physician
Advisor and Team Lead for a revenue cycle management company providing support for acute care hospitalization
claims.
Clayton J Olney, DO was a PA and EMT practicing in a Houston suburb for two years prior to medical school. His
DO degree was conferred in 1993 and he completed Pediatric residency and a Neonatal fellowship at the University
of Texas Health Science Center, Houston. He has more than 20 years’ experience in neonatal medicine with
concomitant medical staff leadership experience in Houston, Dallas and Tacoma. In 2017 he accepted a civilian
neonatal appointment that includes training pediatric residents at Madigan Army Medical Center.
Lydia Aguilar-Kirschner, MD earned her medical degree at UNAM in Mexico City, where she did field work in type 2
diabetes and lactose intolerance in populations around Mexico City. She earned a PhD in Genetic Epidemiology
doing work on type 2 diabetes studying the Mexican-American population in the Rio Grande Valley. She recently
retired after working on the basic research of insulin release for almost 4 decades. She is bilingual and has
played a major role in medical outreach to the latinx community on Vashon Island.
Shawn Boeser has worked in international humanitarian emergency response for 16 years, primarily with the United
Nations and as a freelance advisor. In 2018-19, she worked for the UK government Department for International
Development (DFID) in Uganda on Ebola prevention and preparedness. She currently works as an Operations Manager
for a small technology company based in Washington State. She volunteers on the Vashon Island Emergency
Operations Team.
External Advisors
Jay Shendure is the Scientific Director of the Brotman Baty Institute and a Howard Hughes Medical Investigator
and Professor of Genome Sciences at the University of Washington School of Medicine. His research interests
include genomic technologies and their application to human health and disease. He is a principal investigator
of the Seattle Flu Study and currently co-leads the Seattle Coronavirus Assessment Network (SCAN).
John Sirois is a citizen of the Confederated Tribes of the Colville Reservation, John (say’ay’) served much of
his 20-year career within the Colville Tribes’ government in Cultural Preservation and Renewable Energy and
later as Council Chairman and Member. At UCUT, John facilitates the collaborative intertribal committee process
that establishes a strong policy position for the Upper Columbia Untied Tribes. Though John earned a B.A. in
history from Dartmouth College and a Master of Public Administration from the University of Washington, he feels
especially fortunate to have learned traditional ways from his tribal ancestors and elders.
Tootie Tatum has roots in rural Texas and received her PhD from the University of New Mexico. After working on
the Human Genome Project at Baylor College of Medicine, Tootie founded the Clinical Molecular Diagnostics
Laboratory at Texas Tech School of Medicine and the commercial laboratory that is now MicroGenDx. In 2011 she
joined Lawrence Berkeley National Laboratory's Joint Genome Institute where she led the management of
high-throughput DNA sequencing projects. In 2014, she received an MBA from UC Berkeley and founded Blackhawk
Genomics to assist companies entering the genetic testing market. She directs CLIA clinical genetics
laboratories across the United States.
Eric Walker has more than 30 years of experience in leadership, financial and operations management,
institutional capacity building, and systems development in international non-governmental organizations (NGO).
He has served on executive teams of multiple international NGOs working across the international development
sector, including his long-term role as finance and operations leader of the Seattle-based global health NGO
PATH.